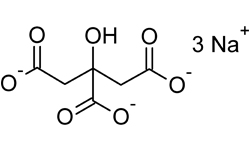
Sodium Citrate
|
SL. NO
|
TESTS
|
STANDARDS
|
||
|
1
|
Characters
|
|||
|
A
|
Description |
White, granular crystals or a white, crystalline powder;
odourless; slightly deliquescent in moist air. |
||
|
B
|
Solubility |
Freely soluble in water; practically insoluble in ethanol
(95%) and ether. |
||
|
2
|
Identification
|
|||
|
A
|
Reaction of Sodium salts | |||
|
i
|
Reaction A
|
A dense white precipitate should be formed.
|
||
|
ii
|
Reaction B
|
No precipitate should be formed.
|
||
|
B
|
Reaction of Citrates | |||
|
i
|
Reaction A
|
A white precipitate should be formed which should be
soluble in 6M acetic acid. |
||
|
ii
|
Reaction B
|
A violet colour should be produced which should change
to violet-blue. |
||
|
3
|
Appearance of Solution
|
Solution should be Clear and Colourless
|
||
|
4
|
Acidity or Alkalinity
|
Not more than 0.5 ml of 0.05M sulphuric acid or 0.1M sodium hydroxide should be required. |
||
|
5
|
Arsenic
|
Not more than 2 ppm.
|
||
|
6
|
Heavy Metals
|
Not more than 10 ppm.
|
||
|
7
|
Chloride
|
Not more than 100 ppm
|
||
|
8
|
Oxalate
(as anhy. oxalic acid) |
Not more than 300 ppm.
|
||
|
9
|
Sulphate
|
Not more than 150 ppm.
|
||
|
10
|
Tartrate
|
No crystalline precipitate should be formed.
|
||
|
11
|
Readily Carbonisable Substances
|
The solution should not be more intensely coloured than
reference solution YS2 or GYS2. |
||
|
12
|
Water
|
Between 11.0% and 13.0%
|
||
|
13
|
Assay
|
Between 99.0% and 101.0%
|
||