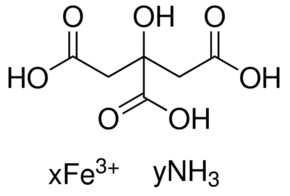
Iron & Ammonium Citrate
|
SL. NO
|
TESTS
|
STANDARDS
|
||
|
1
|
Characters
|
|||
|
A
|
Description |
Thin, transparent, dark red scales or granules or a brownish red powder; odourless; deliquescent in moist air and is affected by light.
|
||
|
B
|
Solubility |
Very soluble in water; practically insoluble in ethanol (95%).
|
||
|
2
|
Identification
|
|||
|
A
|
Reaction of Ferric salts | |||
|
i
|
Reaction A
|
An intense blue precipitate should be produced which should be insoluble in dilute hydrochloric acid.
|
||
|
ii
|
Reaction B
|
The red colour should disappear.
|
||
|
ii
|
Reaction C
|
A stable green colour should be formed.
|
||
|
B
|
Reaction of Citrates | |||
|
i
|
Reaction A
|
A white precipitate should be formed which should be soluble in 6M acetic acid.
|
||
|
ii
|
Reaction B
|
A violet colour should be produced which should change to violet-blue.
|
||
|
3
|
Arsenic
|
Not more than 4 ppm.
|
||
|
4
|
Lead
|
Not more than 30 ppm. | ||
|
5
|
Zinc
|
Not more than 50 ppm.
|
||
|
6
|
Free Ferric Compounds
|
No blue precipitate should be formed.
|
||
|
7
|
Chlorides
|
Not more than 0.25%
|
||
|
8
|
Sulphate
|
Not more than 0.3%
|
||
|
9
|
Assay
|
Between 20.5% and 22.5% (of iron, Fe)
|
||